Explain the Difference Between Crystalline and Amorphous Solids
Is C_60 an electronically- or structurally- driven magic number. Amorphous solids on the other hand are thought to be liquids at all temperatures.
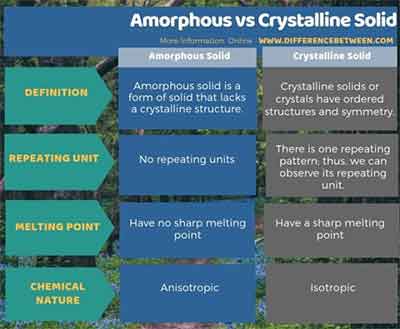
Differences Between Amorphous Crystalline Solids
Amorphous solids dont have an orderly structure and the distance between particles and their intermolecular forces vary.

. But both are polar. Crystals have a specific geometric shape with definite edges. Amorphous solids are isotropic.
While amorphous solids have a. They have an irregular shape. The other main category of solids is called amorphous.
In crystalline solids constituent particles atoms molecules or ions arrange in a three-dimensional periodic manner. Amorphous solids do not have definite melting points but melt over a. Difference between Crystalline and Amorphous.
Crystalline solids have sharp melting points that is they change into liquids at definite temperatures. Are nanomaterials crystalline or amorphous. This is because on being heated they do not abruptly change into liquids but instead soften and start flowing in a semisolid form.
Explain the difference between an amorphous and a crystalline solid. Diamond is an example of Crystalline Solid whereas plastic is an. One major difference between crystalline and amorphous solids is that crystalline solids have a precise melting point.
Non-crystalline solids do not have a consistent arrangement of particles. They have short range order of regular pattern of arrangement of constituent particles. They have only short-range order in the arrangement of constituent particles.
Explain the difference between an amorphous and a crystalline solid. A Amorphous solids has moleculesatoms not arranged organized in a definite lattice patternShows short order of definite patte. Consider a game of checkers.
Crystalline solids have a definite shape with orderly arranged ions molecules. They do not have an ordered arrangement resulting in irregular shapes Melting Points Crystalline Solids They have a sharp melting point. They have a regular and ordered arrangement resulting in a definite shape.
In comparison amorphous solids have no such. They have long range order of regular pattern of arrangement of constituent particles. Amorphous solids have no geometry in their shapes Crystalline solids.
They have definite characteristic geometrical shape. Crystalline solids break unpredictably and can produce curved fragments. They have a definite characteristic geometrical shape.
Amorphous solids particles are arranged randomly in no order while crystalline solids particles are arranged in geometric patterns. Are nanomaterials crystalline or amorphous. Difference Between Crystalline and Amorphous Solids Crystals have an orderly arrangement of their constituent particles.
A crystalline solid has a pattern of atoms or molecules that baring defects could repeat infinitelyAn amorphous solid has no such pattern. The key difference between amorphous and crystalline solid is that the crystalline solids have an ordered long-range arrangement of atoms or molecules within the structure whereas the amorphous solids lack ordered long-range arrangement. Difference between amorphous and crystalline solids.
They have irregular shape. Amorphous compounds melt gradually across a temperature range whereas crystalline solids have sharp melting points. Crystalline structures have a definite arrangement of particles.
The difference between crystalline and amorphous is mainly based on the structure. They do not have regular arrangement. Crystalline Solids Particles are arranged in a repeating pattern.
The distance between particles as well as the intermolecular forces are constant. Crystalline solids are anisotropic while amorphous ones are isotropic. This means that they have no definite edges.
Atoms are arranged in regular 3 dimension. Melting points of Crystalline and Amorphous. No particular melting point.
So non-crystalline solids are amorphous solids. We review their content and use your feedback to keep the quality high. As a result crystalline solids have specific temperatures of fusion whereas amorphous solids do not.
In physics and chemistry amorphous is a term used to describe a solid which does not exhibit crystalline structure. Key differences between amorphous and crystalline Structure of Crystalline and Amorphous. View the full answer.
We can classify solids into two as crystalline and amorphous depending on the atomic level arrangement. However now glass is considered to be one type of amorphous solid. 9 rows The difference between amorphous and crystalline solids are discussed below.
Amorphous solids have a lattice structure. Amorphous solids are softer and more pliable than crystalline ones. They are polar and amorphous.
Explain the difference between an amorphous and a crystalline solid. While there may be local ordering of the atoms or molecules in an amorphous solid no long-term ordering is present. While crystalline solids are well ordered at the atomic level with each atom or molecule inhabiting a specific point on a lattice amorphous solids are disordered at an atomic level with the atoms or molecules held together in a completely random formation.
Solids and liquids cannot be compressed as much as gases the molecules are too close together what is the major difference between a crystal and an amorphous solid. The former has a sharp melting point and is brittle. They are true solids.
Amorphous Solids Particles are arranged randomly.
Difference Between Amorphous And Crystalline Solids Definition Structure Properties Examples

Difference Between Crystalline And Amorphous Solids Solution Pharmacy
Difference Between Crystalline And Amorphous Difference Between
0 Response to "Explain the Difference Between Crystalline and Amorphous Solids"
Post a Comment